FACTS & FALLACIES ABOUT PROBIOTICS
Human beings eat food to derive energy and nutrition for the the purpose of sustenance, growth and reproduction. In fact, this is true for all the living systems, such as plants, microorganisms and animals. In the case of animals (including humans), food is partially digested in the alimentary canal, mouth and stomach and finally in the intestine, where the partially digested food is utimately metabolized by billions of microorganisms working simultaneously and synergistically It has been said that there are more bacteria in one person at one time than there are people on this earth. Fortunately, however, less than one percent of all the known types of microoganisms are undesirable or pathogenic. In the case of animals (including humans), food is partially digested in the alimentary canal, mouth and stomach and finally in the intestine, where the partially digested food is utimately metabolized by billions of microorganisms working simultaneously and synergistically It has been said that there are more bacteria in one person at one time than there are people on this earth. Fortunately, however, less than one percent of all the known types of microoganisms are undesirable or pathogenic.
A healthy intestine is one which maintains a critical balance between various groups of these bacteria such as lactobacilli, streptococci, clostridia, coliform and bacteriodes. Any sub-optimal or unhealthy conditions like stress, onset of disease, ingestion of antibiotics and/or medicines, and improper food and rest, and harmful environmental conditions may endanger this fine balance in the intestinal flora, resulting in the reduction of the friendly or beneficial bacteria like lactobacilli and bifidobacteria in the gut.
For centuries the lactic acid bacteria have been used for the preservation of food for human consumption. It has been well documented that certain types of lactobacilli and bifidobacteria are essential or desirable for optimal health. Metchnikoff (1) was perhaps the first researcher, and he concluded in 1908 that the long life span of the Balkans was due to the ingestion of large quantities of lactobacilli and other lactic organisms through fermented foods, which inhibit pathogens and detoxify their system.
For nearly a hundred years research has been conducted on lactobacillic cultures and many amazing facts about their "probiotic" nature have been established. Probiotic refers to those microorganisms which may prevent or reduce the effect of an infection caused by a pathogenic organism. In fact, such probiotic aspects have been consequently related closely to the beneficial, nutritional and therapeutic properties of these organisms.
RESEARCH FACTS ABOUT L. ACIDOPHILUS
At the University of Nebraska research on Lactobacillus acidophilus was started as early as 1925, over 65 years ago. For the past 40 years, scientists headed by the author have worked on L. acidophilus, L. bifidus (now renamed as Bifidobacterium bifidum) and other lactic cultures and have published more than 60 scientific papers on these cultures. They have demonstrated conclusively that there exists considerable differences among different strains of L. acidophilus. In fact, the same strain grown under different conditions would show different properties. They observed that a specially isolated and cultured strain of DDS-1 L. acidophilus grown and produced under specific conditions has properties of great significance for digestion and nutrition and for physiological health and disease.
The beneficial properties the DDS-1 strain of L. acidophilus based on research documented in internationally reputable, refereed journals are as follows:
- Production of enzymes such as proteases, which help digest proteins, and lipases, to digest fat (2,3).
Production of B vitamins which are biocatalysts in food digestion, particularly folic acid and B12 (4,5).
Improvement of the digestibility of food for animals (6).
- Production of the natural antibiotic Acidophilin which has been patented, and Bulgarican has been produced from L. bulgaricus (7,8,9).
- Inhibition of the growth of 23 toxic producing microorganisms (9,10).
Certain yogurt cultures (L. bulgaricus and S. thermophilus) appear to possess great potential as Anticarcinogenic and antitumor properties (11,12).
- Help in the alleviation of lactose intolerance caused by the deficiency of the enzyme lactase. Such L. acidophilus cultures produce significant quantities of lactase which may help digest lactose more fully and thereby reduce the possibility of bad breath, bloating, gas formation and stomach cramps (13,14).
Additional research with Lactobacilli from this laboratory as well as by other scientists has revealed that:
- L. acidophilus, by virtue of their inhibiting gastrointestinal- and uro-pathogens, may help reduce the occurrence of diarrhea and urinary & vaginal infection (15,16, 17).
- L. acidophilus helps enhance calcium metabolism and thus may be related to their hypocholesteremic and anticarcinogenic effect (18,19).
- L. acidophilus helps reduce serum cholesterol levels (18,10).
- L. acidophilus helps alleviate dermatitis and other skin disorders by modifying and improving gastrointestinal microbial balance (21).
- L. acidophilus Aids in the production and/or augmentation of immune bodies and their functions (22,23,24,25).
It is probable that the observed variations in the anticarcinogenic, hypocholesterolytic and antibiotic effects of lactobacilli may be related to the extent of the production and/or activation of immune factors in the animal. In general, lactobacilli lack any antifungal activity. However, the observed beneficial effect of certain lactobacilli on candidiasis under certain conditions may be, in part at least, related to the immunological augmentation or activation in the host.
CHRONOLOGY OF THREE GENERATIONS OF PROBIOTICS:
There were possibly only four or five probiotic microbial supplements on the national market prior to the 1980's. Of these early products I can recall only Lactinex and Setebaid. Although Setebaid was fairly expensive, it had no live organisms, and I don't think it is on the market any more. Then by the early 1980's several newer L. acidophilus products appeared on the market in the form of liquid, powder, capsules and tablets. As far as it could be ascertained, American Culture and Enzyme Systems (later renamed as Nebraska Cultures) was perhaps the first to come out with a true quot;Non-Dairyquot; acidophilus (containing no milk components such as lactose, casein, or any other milk protein). By mid 1980 numerous other acidophilus products and multi- microbial supplements were developed - like acidophilus with bifidus, faecium, bulgaricus, rhamnosus, lactis, and others. More recently on the other hand, there have appeared products containing E. coli, Bacillus laterosporus and Bacillus sphaericus and others.
ADDITIONAL CRITERIA OF FACTUALLY BENEFICIAL ACIDOPHILUS
To assure that L. acidophilus possess these nutritional and therapeutic properties, it must implant and multiply rapidly in the gut to avoid its being expunged entirely. Hence for gut inhabitation L. acidophilus must not only be able to tolerate and pass through the high stomach activity (low pH), but also be able to grow and proliferate at physiological levels of bile salts and adhere to the intestinal epithelial cells.
L. acidophilus strains are known to differ tremendously in their ability to grow in the presence of bile salts. Bile salts, produced by the gall bladder, are essential in helping to emulsify fat before it can be digested in the intestine (16). L. acidophilus DDS-1 has been reported to be highly resistant to several commonly known antibiotics like penicillin, streptomycin, aureomycin, etc. Such antibiotic resistance of L. acidophilus DDS-1 is of paramount importance because it can be taken while or soon after an individual has been on antibiotic therapy. Common antibiotic therapy not only kills the pathogenic bacteria but also kills "friendly bacteria" like lactobacilli and streptococci and may upset gastrointestinal microbial balance. The DDS-1 strain of L. acidophilus may thus help in restoring the optimal microbial balance in the gut.
FALLACIES ABOUT ACIDOPHILUS PRODUCTS
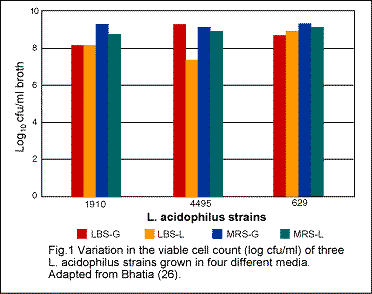
The literature is replete with studies concerning the beneficial role of lactobacilli in general and L. acidophilus in particular. Nevertheless, there exist numerous reports indicating divergent, and sometimes, conflicting observations. It has been established that such conflicting results may very well be due to the different strains used, different methods of manufacture or propagation employed, and of course, due to the different techniques used by different scientists. As indicated earlier even the same strain grown under different conditions may have different properties (26). Similarily, L. acidophilus DDS-1 manufactured by methods other than those used in the research of Shahani may have different characteristics and properties.
 Consequently, all L. acidophilus products on the market are not alike. Some cannot even survive human stomach fluids. Many products contain extremely low levels of living and stable L. acidophilus cells. Some manufacturers give numbers of live cells at the time of formulation, packaging, or bottling, but after manufacturing and storage the number of live cells can drop to almost zero.
Consequently, all L. acidophilus products on the market are not alike. Some cannot even survive human stomach fluids. Many products contain extremely low levels of living and stable L. acidophilus cells. Some manufacturers give numbers of live cells at the time of formulation, packaging, or bottling, but after manufacturing and storage the number of live cells can drop to almost zero.
During the past 20 years or so, as a part of our ongoing research program on probiotics at the University of Nebraska, more than 155 acidophilus products collected from U.S.A. and abroad were examined and enumerated (Table 1). Almost 70 to 80% of the samples did not measure up to numerical claims, and in fact, nearly 50% of the samples did not have even 10% of the claimed number of live microorganisms. For example, if the product was supposed to have 5 billion/gm, it might not have even 500 million/gm. More than 40 to 50% of the product had more than one species of acidophilus. Several products even had microorganisms belonging to a genus other than Lactobacillus. For example, in addition to L. acidophilus they had Streptococcus lactis. Several of the samples even had undesirable or pathogenic organisms present. These are not only our observations; at least two or three papers in scientific journals authored by very renowned microbiologists have reported essentially similar results (27,28). Also, almost all acidophilus products carry promotional material with similar claims without any literature identifying the research work and where it was published, or whether it was published in any peer-reviewed reputable journal.
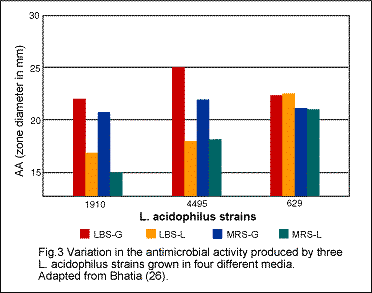
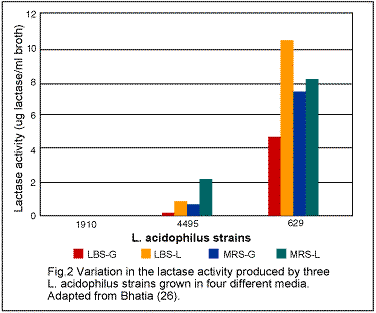 Some of the acidophilus products contain Lactobacillus lactis var rhamnosus (or L. rhamnosus). Although rhamnosus has been reported to be a normal resident of the human gastointestinal tract, little scientific information is available concerning its nutritional importance. Although L. bulgaricus, another food supplement on the market, and one of the two microorganisms (the other being Streptococcus thermophilus) used in the manufacture of yogurt, is considered to be beneficial to the human organism, further research on it should be done. In order for any microorganism to continue to play any beneficial role beyond the period of time of its actual ingestion, it has to implant itself so that it can continue to multiply in the intestinal wall, like L. acidophilus. There appears to be some controversy about the implantation of L. bulgaricus in the human body. Some of the acidophilus products contain Lactobacillus lactis var rhamnosus (or L. rhamnosus). Although rhamnosus has been reported to be a normal resident of the human gastointestinal tract, little scientific information is available concerning its nutritional importance. Although L. bulgaricus, another food supplement on the market, and one of the two microorganisms (the other being Streptococcus thermophilus) used in the manufacture of yogurt, is considered to be beneficial to the human organism, further research on it should be done. In order for any microorganism to continue to play any beneficial role beyond the period of time of its actual ingestion, it has to implant itself so that it can continue to multiply in the intestinal wall, like L. acidophilus. There appears to be some controversy about the implantation of L. bulgaricus in the human body.
Lately, another probiotic product from Germany named Mutaflor, claimed to have been derived from E. coli or coliform, has appeared on the market. Although in general coliform bacteria have been considered rather undesirable, studies with Mutaflor reportedly have shown it to possess some beneficial attributes indicating possible differences among different strains of coliform organisms. Also, more recent products, such as Microflora, Neoflora or Superflora reportedly contain Bacillus laterosporus, Bacillus sphaericus, some amino acids, some electrolyte salts and vitamins. While amino acids, vitamins and electrolytes may play a nutritional role, and some studies have demonstrated B. laterosporus to possess certain probiotic attributes, there does not seem to exist any scientific information about B. sphaericus. Even Bergey's Manual (which is considered to be the Microbiologist' "Bible") does not show B. sphaericus to have any probiotic properties. In actuality it may be dangerous or prove to be harmful under certain conditions.
Following any antibiotic therapy or use, the number of beneficial or desirable gastrointestinal microflora (such as acidophilus or bifidobacteria) goes down and ratio of the undesirable to desirable bacteria goes up. This is because the desirable bacteria like acidophilus and bifidobacteria, being Gram +ve, are more severely killed by most of the commonly used antibiotics. It is at this time that supplements of desirable bacteria are most needed. If, instead of taking supplementary bacteria that have been proven to be desirable, one takes supplements of bacteria of unproven benefit, one may be actually helping the undesirable organisms to continue their predominance.
MANUFACTURE OF FREEZE DRIED DDS-1 STRAIN OF L. ACIDOPHILUS
 The DDS-1 strain of L. acidophilus is manufactured by an exclusive, unique process involving growth in a well-defined and highly nourishing medium for this special strain. In the manufacturing process the microorganisms are concentrated first by removing unspent liquid medium by sedimentation, ultrafiltration, reverse osmosis, and/or centrifugation. To the intact cell concentrate is then added a definite cryoprotectant before freezing to prevent "freezer damage" to the bacteria (29). The DDS-1 strain of L. acidophilus is manufactured by an exclusive, unique process involving growth in a well-defined and highly nourishing medium for this special strain. In the manufacturing process the microorganisms are concentrated first by removing unspent liquid medium by sedimentation, ultrafiltration, reverse osmosis, and/or centrifugation. To the intact cell concentrate is then added a definite cryoprotectant before freezing to prevent "freezer damage" to the bacteria (29).
Following freezing, the mass is freeze-dried in a specially designed unit. The final product is then subjected to fine screening and quality control involving at least 15 to 20 tests. When the product passes all the rigorous tests, it is then mixed with a natural stabilizer to prevent the loss of its viability during packaging, shipping, storage, marketing and consumption. As far as is known, the visibility of the cells is not damaged at all during sedimentation, ultrafiltration, reverse osmosis or centrifugation, unless the processing equipment is faulty and/or the processor is not properly trained.
STABILITY
The DDS-1 strain of L. acidophilus is highly stable even under adverse conditions of manufacture and storage. Normally, microorganisms such as L. acidophilus are affected adversely by heat, moisture or humidity, light and air. The unique process of manufacturing DDS-1 coupled with the addition of a suitable cryoprotectant and specially designed natural stabilizer protect the microorganisms against moisture, light, heat and oxygen (from air), providing a stability unsurpassed as far as is known.
RESPONSIBILITIES OF THE FOOD SUPPLEMENT ADVOCATE
As indicated above, all the different acidophilus products are not alike in that they do not have similar properties. Similarly, a known strain of L. acidophilus, even L. acidophilus DDS-1, manufactured by different methods, will not have similar properties and stability.
There exist numerous food supplements on the market which do not meet the standards claimed on their labels. The consumer has every right to demand that the product one purchases actually meets the standards or the claims made for that product. It is therefore a serious responsibility of the health professional, and of the health food-store as well, to make sure that the manufacturer and/or the supplier provides documented proof, preferably published in a peer-reviewed scientific journal or publication, that the claims made for the product were indeed substantiated by tests or research done with that product.
| TABLE 1. OVERALL SURVEY EVALUATION |
| Percentage |
Description |
| 70-90 |
Below numerical claims |
| 20-40 |
Below 10% of numerical claims |
| 40-60 |
More than one species |
| 10-20 |
Non-acidophilus Lactobacillus |
| 1-5 |
Undesirable Microorganisms |
| 1 |
No acidophilus, not even Lactobacillus |
 Facts &
Fallacies Bibliography Facts &
Fallacies Bibliography
Top of Page
|
|

© Copyright 1997 Nebraska Cultures, Inc. All rights reserved.
|
|


